Medidee Knowledge Hub
Highlights
WEBINAR
2024 IVDR Sprint – Companion Diagnostics (CDx) – Critical Areas and Implications for Successful Development and Launch in the EU
In this recorded webinar, learn some of our first-hand industry expertise, helping you stay attuned to the latest industry developments, challenges and advancements specifically related to Companion Diagnostics and IVDs.
WEBINAR
2024 IVDR Sprint – How to Adopt a Proactive Approach to Ensure Timely Compliance with the Applicable Deadlines
In this recorded webinar, acquire insights into the In Vitro Diagnostic Regulation (IVDR) landscape, and comprehend how IVDR requirements aid organizations in fulfilling legal and regulatory obligations associated with In Vitro Diagnostics (IVD) compliance in the EU market.
WEBINAR
2024 IVDR Sprint – The Clinical Challenge:Addressing Increased Evidence Requirements in the EU IVDR Era
In this recorded webinar, gain an in-depth comprehension of IVDR requirements relevant to Performance Evaluation, and understand the importance and benefits of conducting a Performance Evaluation as early as possible in the design and development of a new IVD, or in preparation for the transition of a legacy device to IVDR.
WEBINAR
2024 IVDR Sprint – How to Deal with Regulatory Requirements when Collecting Clinical Performance Data
In this recorded webinar, gain an understanding of the clinical performance study prerequisites outlined in the In Vitro Diagnostic Regulation (IVDR), improve comprehension of utilizing EN ISO 20916:2024 to ensure clinical performance studies align with IVDR compliance standards and learn best practices and pitfalls to avoid during the preparation and execution of a clinical performance study, drawn from our expertise as CRO requirements.
WEBINAR
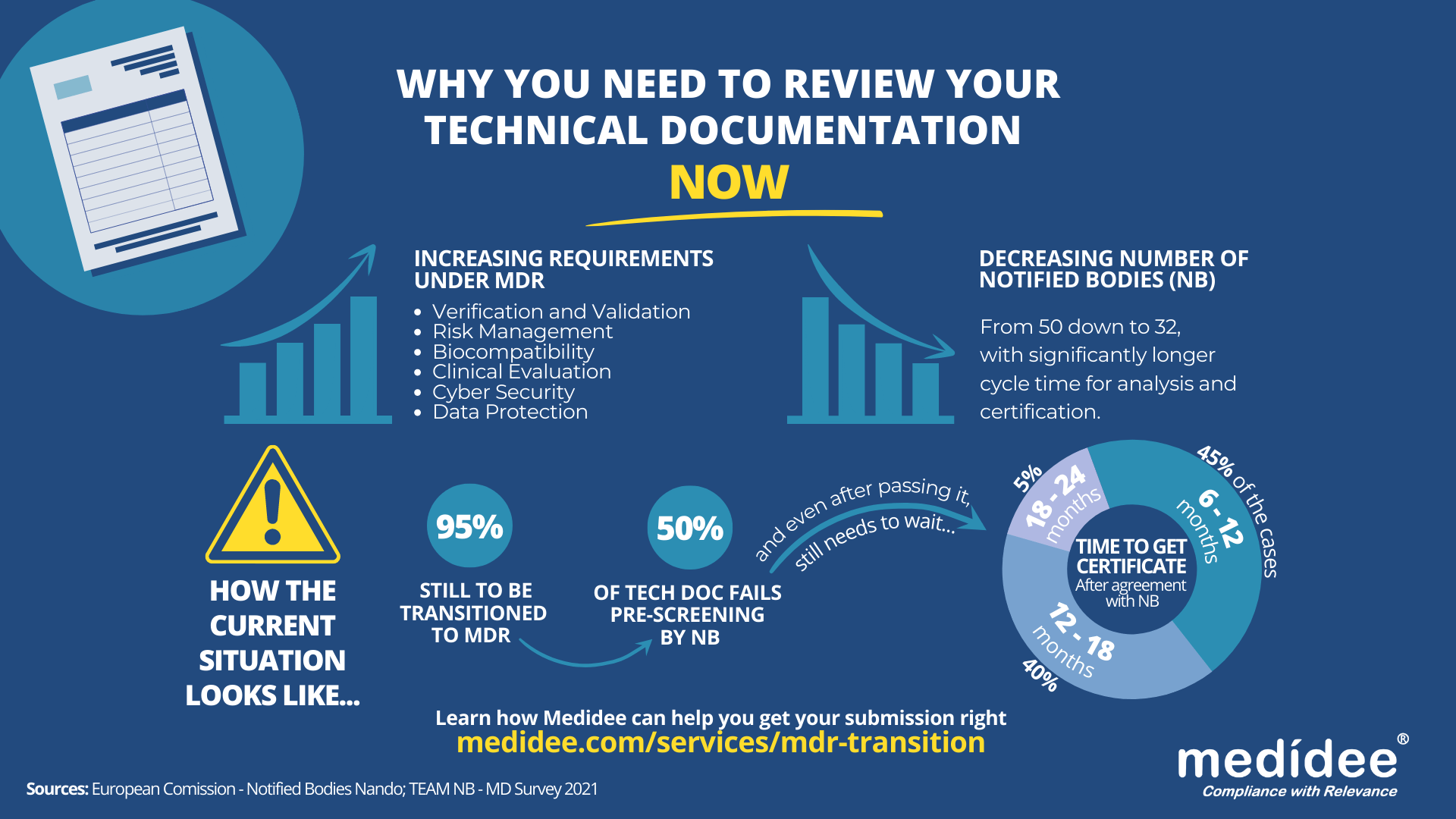
How to build compliant Technical Documentation (TD) under MDR?
To expedite the Technical Documentation review process by the Notified Body and avoid extra costs and delays (which potentially could make your company miss the May 2024 deadline), your technical documentation submission needs to be right the first time!
ARTICLE
Artificial Intelligence (AI), General Data Protection Regulation (GDPR) and Cybersecurity: 10 Misconceptions about Medical Device Software
Medical Device Software (MDSW) is a growing, fast-evolving industry. The purpose of this article is to address some of the most common (and not always right) assumptions and provide useful and truthful information about the process of reaching the conformity assessment under MDR, for successfully placing an MDSW on the market.
April 12, 2023
[TECH LETTER] Substance-Based Products
June 29, 2022
[WEBINAR] Clinical Evaluation Reports
February 11, 2022
[ARTICLE] IEC 60601 standard series
October 7, 2021
[WEBINAR] EU or US approval?
September 14, 2021
[WEBINAR] Performance Evaluation Plan & Report
March 24, 2021