[ARTICLE] In-Vitro Diagnostic Medical Devices Q&A: State of the Art in IVDR
As required by the IVD Medical Device Regulation (EU) 2017/747, a device performance evaluation plan must contain a description of the state of the art pertaining to the IVD device under evaluation.
All relevant aspects related to the evaluation of the performance of a device must be weighed against the current state of the art. These include:
- the demonstration of scientific validity, device analytical performance and clinical performance;
- the evaluation of the risk control measures implemented;
- the technical safety of the device;
- the IVD’s expected clinical benefit.
In other words, the clinical evidence must be such as to scientifically demonstrate, by reference to the current state of the art of medicine, that the IVD medical device has a positive risk/benefit ratio. Cited 20 times in the IVDR (EU) 2017/747I, for example in relation to the design of in vitro diagnostic medical devices, as well as risk management, performance evaluation and post-market surveillance, it is clear that state-of-the-art assessment plays a central role in the overall life cycle of a device and not just in relation to performance evaluation.
In this blog post, we provide comprehensive answers to some of the most common questions about the state of the art in IVD medical devices.
What does “State of the Art” mean?
Manufacturers are expected to regularly, throughout the device life cycle, review and evaluate the evolution of the state of the art for their IVD medical devices.
However, there is no formal definition of state of the art in the IVD regulations. MDCG 2022-2 published in February 2022 and entitled “Guidance on general principles of clinical evidence for In Vitro Diagnostic medical devices (IVDs)” defines state of the art as:
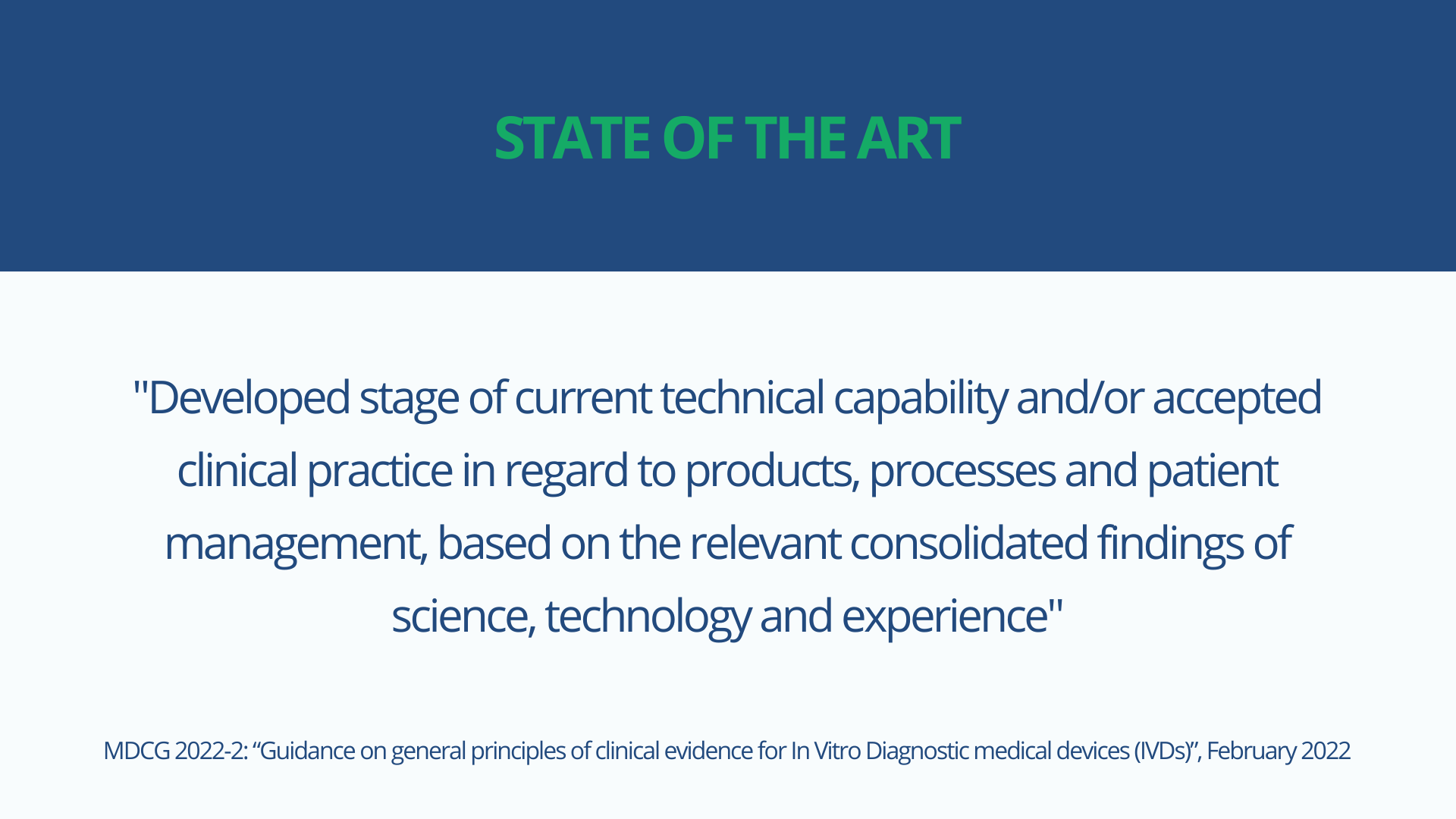
It further clarifies that the state of the art embodies what is currently and generally accepted as good practice in technology and medicine, which does not necessarily represent the most technologically advanced solution.
Therefore, state of the art should be understood as the accepted standard clinical practice in a particular medical field, which is not necessarily the most advanced technology, and should be used to demonstrate the acceptability of the IVD medical device based on that current knowledge.
As per IVDR requirements, the state-of-the-art description shall include the identification of existing relevant standards, CS, guidance or best practices documents. Furthermore, the state-of-the-art assessment may contain a mix of:
- what is currently considered best clinical practice for diagnosing/detecting or monitoring a particular condition/disease;
- what biomarkers are commonly accepted;
- what technology is most commonly used to measure those biomarkers.
As the state of the art evolves over time, such an evaluation must be conducted regularly to reflect the most recent knowledge.
To give a concrete example of the possible evolution of the state of the art over time: for the diagnosis of myocardial infarction, the creatine-kinase-MB assays were for years considered the standard of care. In the last 10 years, however, these assays have been replaced in routine clinical practice by assays measuring cardiac troponin and high-sensitivity cardiac troponin. However, the technology for detecting these biomarkers, namely immunoassays, remains the same.

When to start the assessment of the state of the art?
The findings of the state-of-the-art assessment will impact several parts of the device’s technical documentation. First, the results of the state-of-the-art assessment may trigger design choices, such as using one particular technology over another or using a specific biomarker or set of biomarkers over others.
In addition, the risk control measures adopted by manufacturers for the design and manufacture of devices must be in line with safety principles, taking into account the generally accepted state of the art. The results of the state-of-the-art assessment will therefore also have an impact on risk assessment and management.
Furthermore, the “state of the art” is not only used to address the clinical evidence of the device, thus being part of the performance evaluation plan, but it is also used to address the safety and performance summary for Class C or Class D devices and is a key component of the post-market surveillance (PMS) requirements. State-of-the-art surveillance is considered a proactive PMS activity and must be covered in the PMS plan.
Therefore, a state-of-the-art assessment must be conducted very early during the development of the device and must be regularly reassessed and updated, when necessary, through the PMS activities and during the entire life cycle of the device. The design and risk/benefit ratio of the device should be considered in light of the results of the most recent state-of-the-art evaluation.
How should the state of the art be assessed?
The assessment of the state of the art involves a systematic and methodological approach to the literature review, which requires time and resources.
Acceptable inputs for assessing the state of the art for an IVD may include:
- Published common specifications such as those published for the assessment of the performance of SARA-CoV 2 assays and for other class D devices
- Standards used for the same or similar devices
- Clinical guidance documents and medical textbooks
- Best practices, as used in other devices of the same or similar type
- Expert consensus documents produced by professional medical associations
- Publications from regulatory authorities or additional information for similar other products
- Recommendations from medical or laboratory associations
- Comparison of the benefits and risks of the device under development with the benefits and risks of similar devices available on the market
- Peer-reviewed literature, such as review articles on relevant condition/disease managed by the device, biomarkers or device technology.
The assessment of the literature must be conducted using explicit and systematic data appraisal and collection. For IVD medical devices there are currently no specific guidance documents describing how such a methodological review of the literature needs to be conducted to achieve an adequate state-of-the-art assessment.
The guidance document MEDEV 2.7/1 rev 4 entitled “Clinical Evaluation: A Guide for Manufacturers and Notified Bodies under Directives 93/42/EEC and 90/385/EEC” provides standards of good practice for methodological literature reviews, offering general instructions on literature searching and describing methods for developing a protocol for conducting a literature review.
Although this guidance document does not address in vitro diagnostic devices per se, it nevertheless represents the gold standard for methodologically reviewing the state of the art and can be used by manufacturers of in vitro diagnostic devices for this purpose.
It is important that manufacturers document the databases used for the literature search, as well as the applied search strings and keywords, and that identified articles are appraised using traceable and systematic inclusion/exclusion criteria.
Conclusion
In conclusion, the assessment of the state of the art is essential because it is needed to confirm the clinical benefit of the device, to justify the positive risk/benefit ratio of the IVD, and also to guide design choices and risk mitigation.
State-of-the-art evaluation is a living concept, which means that it must be evaluated throughout the life cycle of the device, with the clinical benefit of the device always being updated in light of the most recent state-of-the-art evaluation.
Most manufacturers are aware of the latest state of the art regarding their IVDs, but this is not always documented in a systematic and methodological way in the device’s technical documentation. This can lead to unnecessary delays and an additional burden on resources when the device undergoes conformity assessment by a Notified Body.
It is therefore recommended that a methodological assessment of the state of the art be performed and documented early in the life cycle of the device and updated regularly to reflect the latest knowledge in the relevant medical field.
How Medidee can help
Medidee’s IVDR experts collaborate with your team to design and implement a strategy that is tailored to your specific challenges and needs, for a well-managed transition to IVDR.
Contact us to get started now!
This article was written by Dr Silvia Anghel and Dr Julianne Bobela.
