At the Intersection of Different Regulations
Combination Products combine two or more regulated medical products, each of which, when considered individually, would be governed by different regulations. As a result, the regulatory framework of Combination Products is complex, as it often requires the involvement of different Regulatory Authorities (in the EU, Competent Authorities and/or Notified Bodies; in the US, different FDA centers).

Identifying the Primary Mode of Action of your Product
As a general rule, the key criterion defining how a Combination Product is regulated is its Primary Mode of Action (PMOA). For novel Combination Products, the identification of the PMOA may be challenging, especially for the so-called “borderline cases”. Medidee supports you in defining the regulatory pathway to market of such products, and in taking advantage of the relevant programs, such as the Request for Designation (RFD) or the pre-RFD.
Medidee’s Expertise
Medidee provides support for drug-led Combination Products, such as pre-filled syringes, pen injectors, patch-based delivery systems, and for device-led Combination Products, such as drug-eluting stents.
For Combination Products that are regulated as drugs, Medidee provides support in integrating the regulatory requirements of the “device part”, for example by preparing the Technical Documentation for submission to the relevant Regulatory Authority.

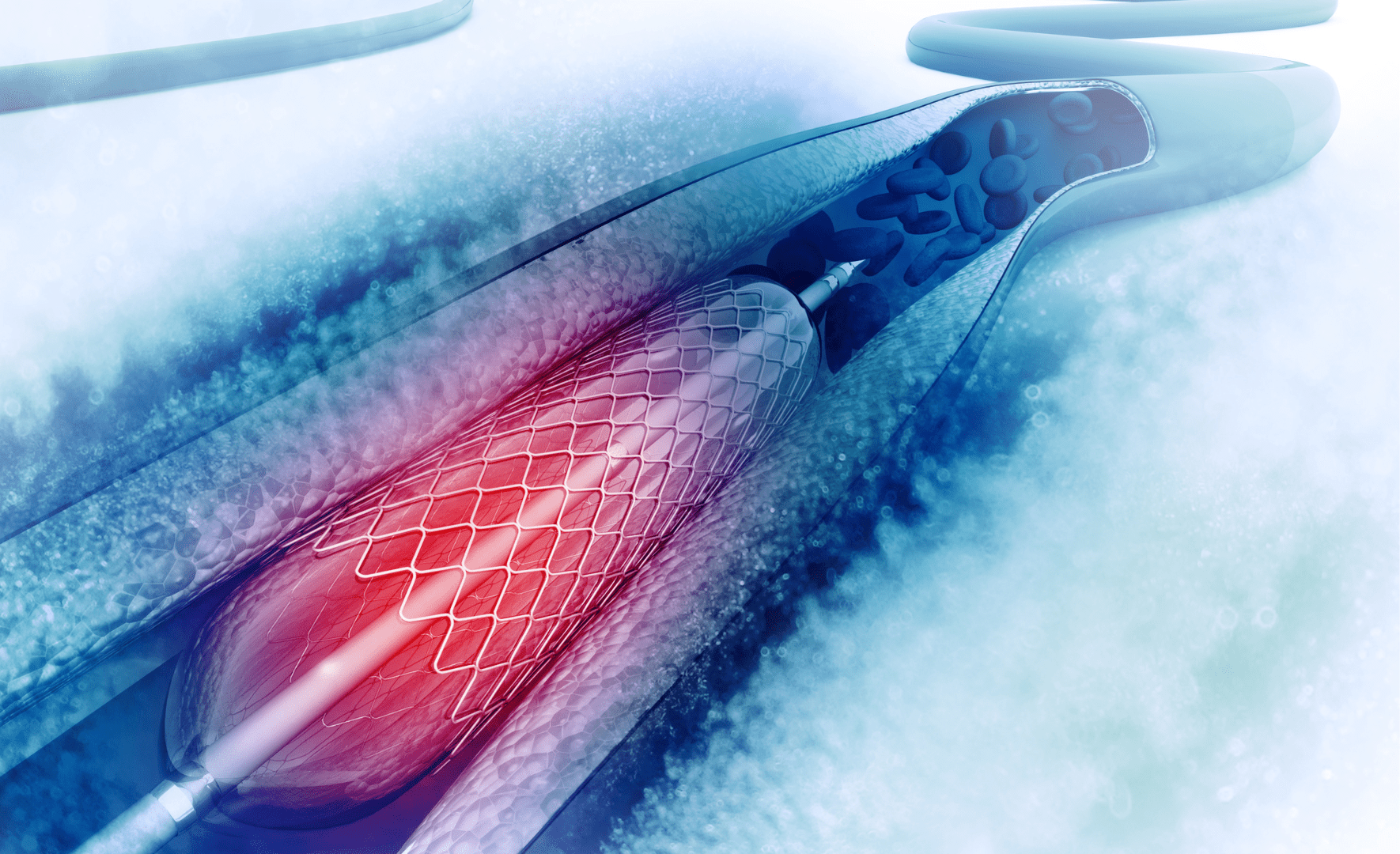
Article 117 of the MDR ((EU) 2017/745) has significantly impacted the regulatory approval process of drug-delivery systems, requiring in most cases the involvement of a Notified Body. Medidee consultants support you in preparing the submission and in the interactions with the Notified Body to obtain the Notified Body Opinion.
For Combination Products that are regulated as medical devices, Medidee provides its expertise across all product development stages.
Medidee's solutions for manufacturers of Combination Products
Covering different types of combination products and supporting access to different markets, this is Medidee’s comprehensive service offering: