[ARTICLE] Electrical Safety: How changes to IEC 60601 Series trigger additional testing for your device (IEC 60601-1, Edition 3.2)
This article is the first one of a series about IEC 60601. It contains insightful information about IEC 60601-1 Edition 3.2, with a similar structure as the second article.
The latest amendment of IEC 60601-1 for medical electrical equipment – Part 1: General requirements for basic safety and essential performance (IEC 60601-1 Edition 3.2) was published on 2020-08-20 by the International Electrotechnical Commission (IEC). Edition 3.2 notably addresses issues raised by national committees and questions submitted to IEC/SC 62A/Working Group 14 with regards to previous version 3.1.
Since the 30th of May 2022, the United States Food & Drug Administration (FDA) has listed IEC 60601-1 3.2 Edition for Medical Electrical Equipment – Part 1: General requirements for basic safety and essential performance as a recognized standard (FDA recognition number 19-46). IEC 60601-1 3.2 Edition will replace the current listed IEC 60601-1 3.1 Edition (FDA recognition number 19-4) by the 17th of December 2023. Until this date, FDA will accept declarations of conformity referring to IEC 60601-1 3.1 Edition, in support of premarket submissions.
To date, no announcement relative to an official transition period for the applicability of Edition 3.2 or to its harmonization under the Medical Device Directive (93/42/EEC) or Medical Device Regulation (EU 2017/745) has been issued in the official journal of the European Union. As per the list of recognized consensus standards of the Food & Drug Administration (FDA), ES60601-1:2005/(R)2012 and A1:2012, C1:2009/(R)2012 and A2:2010/(R)2012 (Consolidated Text) are currently listed.
In the absence of any official communication within the European Union on the matter, it is anticipated that manufacturers will be granted a transition period of 3 to 4 years after the date of publication, before they will be expected to demonstrate compliance with the applicable new requirements introduced by the amended standard.
Medidee has observed that manufacturers of electrically driven active medical devices are commonly unaware of the latest revision of this standard and consequently of the new requirements it entails. This article aims to raise awareness of this amendment of IEC 60601-1, to summarize the main changes compared to the previous version of the standard, and to illustrate through a few examples how these changes may affect manufacturers of medical electrical equipment and systems.
What are main the differences between IEC 60601-1 Edition 3.1 and Edition 3.2?
The main clauses affected in IEC 60601-1 Edition 3.2 are:
Clause 2 normative references
Clause 3 terminology and definitions
Clause 7 identification and marking
Clause 8 protection against electrical hazards
Clause 11 protection against excessive temperatures and other hazards
Clause 13 hazardous situations and fault conditions
Clause 14 programmable electrical medical systems (PEMS)
Importantly, the normative references of the standard have also been updated in Edition 3.2. The changes to referenced standards are shown in bold text below:
IEC 60065:2001+AMD1:2005+AMD2:2010 Audio, video and similar electronic apparatus
IEC 60068-2-2:2007 Environmental testing
IEC 60227-1:2007 Insulated cables
IEC 60245-1:2003+AMD1:2007 Rubber insulated cables – general requirements
IEC 60335-1:2010 Household and similar appliances
IEC 60417 Graphical symbols for use on equipment
IEC 60601-1-2:2014+AMD1:2020 Electromagnetic disturbances – requirements and tests
IEC 60601-1-3:2008+AMD1:2013 Radiation protection in diagnostic X-ray equipment
IEC 60601-1-6:2010+AMD1:2013+AMD2:2020 Usability
IEC 60601-1-8:2006+AMD1:2012+AMD2:2020 Alarm systems in medical electrical equipment
IEC 60664-1:2007 Insulation coordination
IEC 60730-1:2014 Automatic electrical controls for household and similar use
IEC 60747-5-5:2007 Semiconductor devices – optoelectronic devices – photocouplers
IEC 60825-1:2014 Safety of laser products
IEC 60065:2001+AMD1:2005+AMD2:2010 Audio, video and similar electronic apparatus IEC 60068-2-2:2007 Environmental testing
IEC 60227-1:2007 Insulated cables
IEC 60245-1:2003+AMD1:2007 Rubber insulated cables – general requirements
IEC 60335-1:2010 Household and similar appliances
IEC 60417 Graphical symbols for use on equipment
IEC 60601-1-2:2014+AMD1:2020 Electromagnetic disturbances – requirements and tests IEC 60601-1-3:2008+AMD1:2013 Radiation protection in diagnostic X-ray equipment
IEC 60601-1-6:2010+AMD1:2013+AMD2:2020 Usability
IEC 60601-1-8:2006+AMD1:2012+AMD2:2020 Alarm systems in medical electrical equipment
IEC 60664-1:2007 Insulation coordination
IEC 60730-1:2014 Automatic electrical controls for household and similar use
IEC 60747-5-5:2007 Semiconductor devices – optoelectronic devices – photocouplers
IEC 60825-1:2014 Safety of laser products
IEC 60851-3:2009 Winding wires – test methods mechanical properties
IEC 60851-5:2008 Winding wires – test methods electrical properties
IEC 60950-1:2005+AMD1:2009+AMD2:2013 Information technology equipment
IEC 61058-1:2000+AMD1:2001+AMD2:2007 Switches for appliances
IEC 62133 Secondary cells and batteries
IEC 62133-2 Secondary cells and batteries (Lithium systems)
IEC 62304:2006+AMD1:2015 Medical device software – software life cycle processes
IEC 62368-1:2018 Audio/ video, information, and communication technology equipment
ISO 7010:2019 Graphical symbols
ISO 11135-1:2007 Sterilization of health care products – Ethylene oxide
ISO 11137-1:2006 Sterilization of health care products – requirements for validation
ISO 13857:2008 Safety of machinery
ISO 14971:2019 Medical devices – application of risk management
ISO 15223-1:2016 Medical devices – symbols used with medical device labels
ISO 17665-1:2006 Sterilization of health care products – requirements for validation (moist heat)
ISO 80000-1:2009 Quantities and units
What do these changes mean in practice?
Due to the technological progress and resulting technical changes, the standards to ensure a safe and effective medical device needs to develop too (state of the art). To comply with IEC 60601-1 3.2 edition the latest editions of applicable process, particular, collateral, and component standards need to be considered by the medical device applicant.
Medical device manufacturers are advised to take advantage of the transition period to perform a gap analysis of their technical documentation (TD) against Edition 3.2 and update their documentation accordingly. Once potential gaps have been cleared, a new test report according to IEC 60601-1:2005+AMD1:2012+AMD2:2020 Edition 3.2 can be requested from an external ISO/IEC 17025 accredited testing laboratory. If the current IEC 60601-1 test report uses CB-Scheme as test scheme, it should be kept in mind that according to IEC Operational Document OD-2037 clause 3.1, a new Certification Body (CB) test certificate shall be issued with a new CB test certificate number. Thanks to the fact that now the TD will have been revised to comply with technical state of the art, a smooth certification process will be assured.
Let’s focus on the changes to IEC 60601-1 clause 8 – protection against electrical hazards and clause 14 – programmable electrical medical systems. One noticeable change to clause 8 concerns the introduction of the requirement to measure the voltage of all accessible conductive parts of the Signal Input/ Signal Output (SIP/SOP) or power output connectors to earth for medical electrical equipment which is equipped with SIP/SOP connectors or separate power supply output connectors. Depending on whether the voltage is greater than 60Vdc or 42,4Vac – touch current measurement according to clause 8.7.3 will need to be conducted. For measuring the voltage of the SIP/SOP, please refer to the below-mentioned electrical schematic.
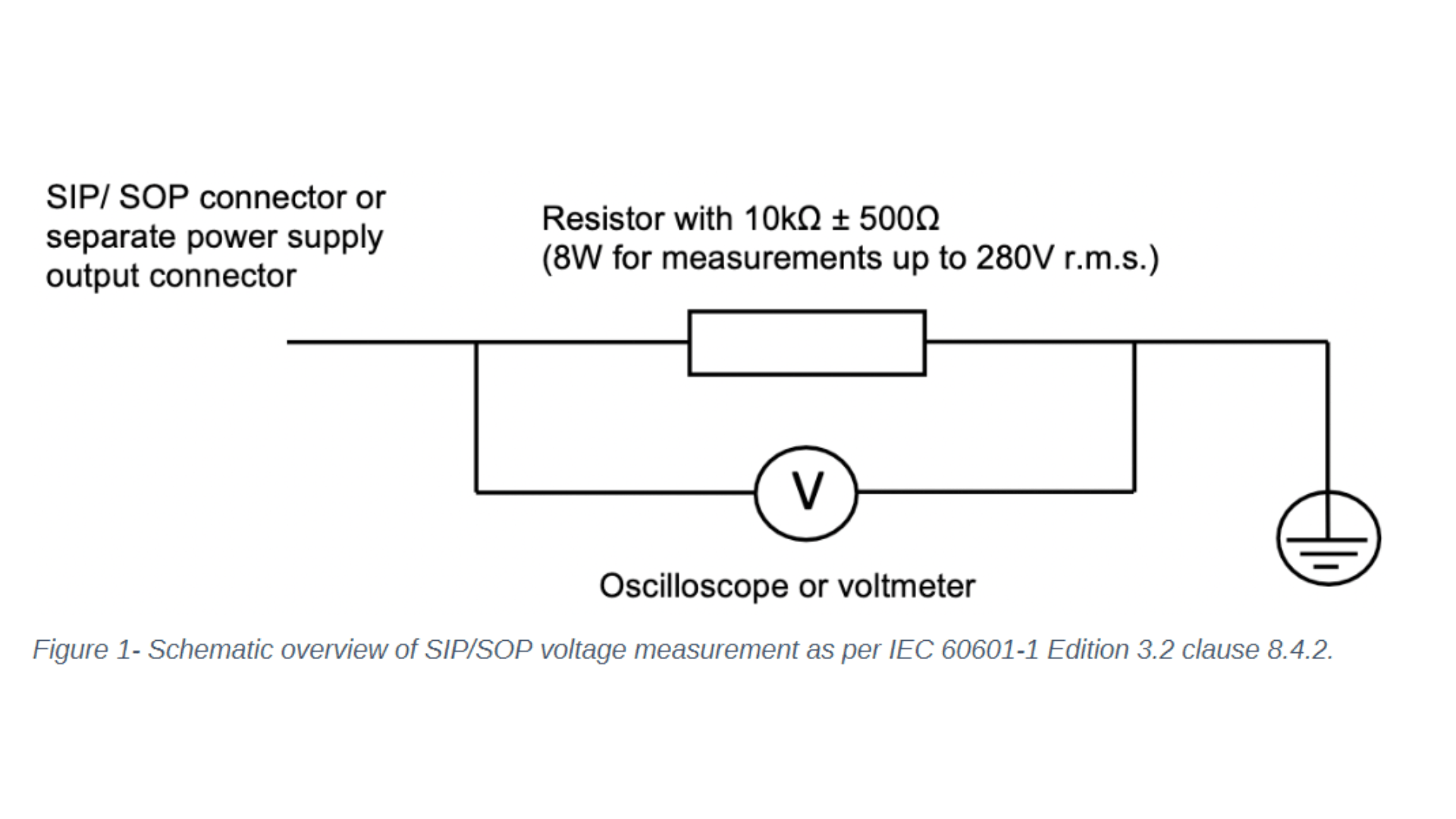
Another noteworthy update of IEC 60601-1 Edition 3.2 affecting clause 8 is the addition of the normative reference to the standard IEC 60747-5-5:2007 Semiconductor devices – optoelectronic devices – photocouplers. Optocouplers which comply with IEC 60747-5-5:2007 or a later edition are considered equivalent to the requirements of 8.8.2 for distances through solid insulation and 8.9.3 for spaces filled by insulation compound. If your medical electrical device or system uses an optocoupler as a solid insulation forming a means of operator protection, the following verification measures apply and need to be conducted:
Air clearance at the outside of the opto-coupler
Creepage distance at the outside of the opto-coupler
Dielectric strength across the opto-coupler
Moreover, Clause 14 Programmable Electrical Medical Systems (PEMS) introduced a direct reference to IEC 62304:2006+AMD1:2015 Medical device software – software life cycle processes. Please keep in mind that the requirements in subclauses 14.2 to 14.12 shall apply to PEMS if the medical electrical equipment uses Programmable Electronics Subsystem (PESS) to provide functionality necessary for basic safety or essential performance or if the application of risk management as described in 4.2 shows that the failure of any PESS leads to an unacceptable risk. Medical device manufacturers are required to evaluate if clause 14 of IEC 60601-1 – and therefore specific clauses of IEC 62304 – are applicable.
How can Medidee support your company?
Our team of electrical safety specialists at Medidee assist you in identifying all areas requiring updates to meet the new requirements introduced by IEC 60601-1 Edition 3.2 through a detailed review of the technical documentation of your medical electrical device or system, as well as provide support in the implementation of applicable new IEC 60601-1 Edition 3.2 requirements. For instance, Medidee reviews and updates the list of critical components and insulation diagram, and process referenced standard-related changes incurred by the update of referenced standards ISO 14971:2019, IEC 62304:2006+AMD1:2015, and IEC 60601-1-6:2010+AMD1:2013+AMD2:2020/ IEC 62366-1:2015+AMD1:2020.
In addition, Medidee supports you in communicating with external accredited testing laboratories and notified bodies, to ensure a smooth certification process of the medical electrical equipment or system.
Contact Medidee today to discuss your needs and how Medidee supports you in addressing them: www.medidee.com/contacts
This article was written by Stefan Staltmayr.

