Agile vs. Regulatory: How the two coexist and contribute to successful Medical Device Software Development
Agile vs. Regulatory: How the two coexist and contribute to successful Medical Device Software Development
Software is gaining relevancy in a broad range of medical devices, as it either enables the control or influence of their operation, or because Software as Medical Device (SaMD) itself has the potential for detection, diagnosis, treatment and alleviation of diverse diseases/disabilities.
General Safety and Performance Requirements (GSPR as per Annex I of the Medical Device Regulation MDR 2017/745 and In Vitro Diagnostics Regulation IVDR 2017/746) are applicable for all SaMD manufacturers. The regulation states in section 17, chapter II of Annex I that:
“17.2. For devices that incorporate software or for software that are devices in themselves, the software shall be developed and manufactured in accordance with the state of the art taking into account the principles of development life cycle, risk management, including information security, verification and validation”
State of the Art
The IEC 62304:2006 – medical device software – software life cycle processes standard is considered the state-of-the-art for software development. Manufacturers shall leverage this standard to comply with the GSPR.
Although this standard is process-oriented and defines a set of specific requirements for manufacturers, Quality Management Systems (QMS), Standard Operational Procedures (SOPs) and lifecycle, it is not essentially different from other standards for software development.
Furthermore, it is not incompatible with Agile methodologies for software (SW) development. In fact, there is a Technical Information Report (TIR45:2012 – Guidance on the use of Agile practices in the development of medical device software) providing some insights and unification of terms for Agile development of SaMD.
In this post, we highlight some differences and correspondences in software development terms between Agile SW and SaMD development regulations, in order to help project teams work better together and channel their efforts in the right direction:
1. Lifecycle
The IEC 62304 requires manufacturers to define the lifecycle and detail the entire process from requirement collection (pre-market) to problem resolution (post-market). Three lifecycles are mentioned in the standard: waterfall, incremental and evolutionary.
Agile is considered incremental/evolutionary and is therefore recognized by the standard as an adequate methodology.
Manufacturers should make it clear, from the beginning, if and when they are using the Agile development methodology. They should always provide this information in the Technical Documentation to enable the software development team and regulatory bodies to understand their development process.
It’s important, however, that the methodology is aligned with the company’s QMS procedures for design and development and embeds the requirements of IEC 62304!
2. The concept of “Done”
When Agile teams have completed an activity, this activity is considered finished and no further actions are expected once it is delivered.
Similarly, section 5.5 of IEC 62304 lists very similar requirements. However, the concept of “Done” should be expanded to include conditions such as:
1) review of requirements,
2) approvals, tester teams and testing conditions,
3) expanded acceptance criteria
4) documentation level required.
In other words, the concepts are similar and the “Done” concept and the underlying process can be widely leveraged to build compliance against IEC 62304, however, additional steps need to be checked before moving an activity to “Done”.
 Techletter
Techletter
Medical Device Software Incorporating Artificial Intelligence
Generating Sufficient Evidence under the MDR
3. Moving to Verification & Validation (V&V)
According to the regulatory requirements as per IEC 62304, integration, hardening and V&V activities need to be performed before the software becomes shippable (final release). However, this is quite difficult for each sprint outcome. To manage it, the Software Development Plan (and most particularly its Verification Plan subpart) must define a “Done is Done” criteria in order to collect a minimum set of increments/epics that could be considered adequate to undergo V&V testing.
Validation according to the regulation is a more complex concept including not only technical performance but also clinical association and clinical validation. For further details on Clinical Validation of Medical Device Software, check our dedicated webpage.
4. Release
Before software is released, all Verification & Validation activities need to be completed and the results evaluated as per the IEC 62304 section 5.8.
Agile methodologies are aligned with these requirements. Indeed, the activities performed at increment level are documented using software development tools, and then at the end of the processes, Agile teams must ensure proper documentation of the consistency and acceptance criteria of all tests that have been performed.
Another requirement set in IEC 62304 is to document known residual anomalies. In Agile, this is created by conducting a review of each increment and searching for the potential known anomalies/bugs to be addressed.
Finally, manufacturers need to ensure that a new release is assessed against the criteria of significant or substantial change, as per Article 120 and Annexes related to Conformity Assessment procedures of the MDR and IVDR. Further details to determine whether an evolution (update/upgrade) might fall under the significant changes as per Article 120 under MDR are found in the MDCG 2020-3 guidance. No guidance related to the concept of substantial change has been published to date.
The examples provided above represent just a thin part of the parallelisms between the Agile development process and IEC 62304 requirements. Based on our experience, most of the software development processes that are IEC 62304 agnostic are very often just a few adjustments away from compliance with the regulatory requirements.
At Medidee, now part of Veranex, we have been working with numerous software development teams, helping them leverage their pre-existing tools, working practices and methodologies to build compliance against IEC 62304, and other related standards, in a pragmatic fashion.
If this is something you are currently working on, do not hesitate to check our full offering of Digital Health Services, or to reach out directly for expert support: https://www.medidee.com/contacts/
This article was written by Dr. Gustavo Hernandez and Dr. Nuria Gresa.
[ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs)
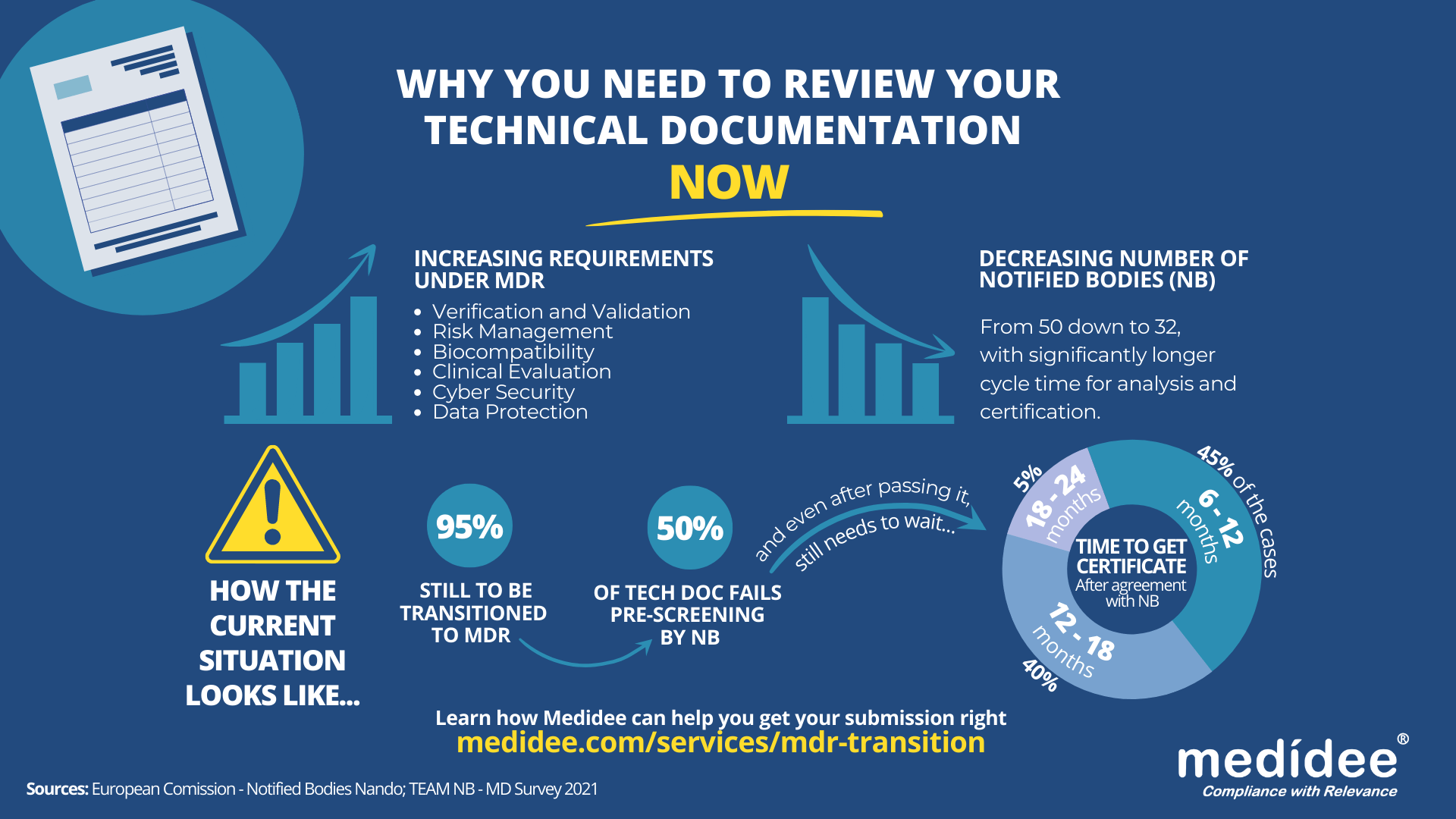
According to the Medical Device Survey 2021 by The European Association for Medical devices of Notified Bodies, Medical Device (MD) companies who have applied for MD Regulation (MDR 2017/754) certification remain a minority (only 26.3% had applied at the end of 2021). A lot of applications are yet to be received!
Bottleneck for Technical Documentation (TD) review by Notified Bodies (NB) is a reality
With increased requirements to comply with MDR and a decreased number of Notified Bodies being able to deliver certificates under those regulations (from 50 down to 32 as of August 1st), it gets longer and longer to obtain a certification.
Currently, from the positive outcome of the Completeness Check, it takes between 6 (low-risk devices) and 24 months (high risk)until the issuance of the MDR certificate. Knowing that certificates issued under the MDD/AIMDD remain valid until May 2024 at the latest, waiting too long before submitting your Technical Documentation can result in having no valid certificate to operate in the market.
How to speed up the certification process: be right the first time!
The requirements under MDR are more stringent compared to previous directives. A thorough gap analysis of your Technical Documentation needs to be performed at first to identify the (possibly) missing documents to demonstrate compliance against all General Safety and Performance Requirements (GSPR). Additional device testing might also be required which will significantly delay the moment of application to a NB.
Indeed, with the implementation of the Completeness Check process, at least 50% of the TD submitted are deemed incomplete, and NB request additional information to start the assessment according to the Medical Device Survey 2021. These numbers are confirmed by two other surveys published very recently by the European Commission and MedTech Europe.
Considering these timelines, you cannot afford to wait for the initial feedback from the Notified Body to discover deficiencies in your Technical Documentation – you really need to be right the first time, avoiding common pitfalls.
8 Technical Documentation pitfalls to avoid at all costs
Having performed many gap analyses regarding TD compliance against MDR, as well as having accompanied several companies through MDR transition, Medidee has a clear vision of the main pitfalls throughout this process. Below are typical examples of which companies should be aware before submission. If you would like to learn more about this topic, don't miss the chance to watch our on-demand webinar:
Pitfall 1: Your TD is not well organized
A clear structure throughout the technical documentation is helpful in ensuring that the reviewing body can clearly understand the contents. The MDR now provides, in Annexes II and III, detailed instructions on what is the minimum content of technical documentation, also defining a specific structure for it.
Pitfall 2: No identifiable thread within your TD
Manufacturers shall maintain traceability from the User Requirements Specification (URS) to the Functional Requirements Specification (FRS), risk analysis, clinical evaluation and the GSPR. This is key to demonstrating full compliance to GSPR.
Pitfall 3: Negligence of Post Market Surveillance data
Manufacturers of devices, even those that have been on the market for many years, need to collect or complete a review of existing Post-Market Surveillance (PMS) data, to be able to cover the requirements related to clinical evaluation, as set out by Article 61 of the MDR. For devices that have previously been placed on the market, this includes, but is not limited to the Post Market Clinical Follow Up (PMCF) data, vigilance data, user feedback and complaints.
Pitfall 4: No anticipation of risk class changes for your device
Although this applies to all MDs, it is particularly true for Software as Medical Device (SaMD). Indeed, the classification rule 11 from MDR, and related MDCG guideline MDCG 2019-11, lead to a class upgrade: 80% of the SaMD which did not need a NB before MDR implementation do require one now. This leads to a tremendous change in the company’s organization to obtain a certificate, while also adding to the above-mentioned bottleneck effect.
Pitfall 5: Not enough precision on how GSPR compliance is reached
Manufacturers must provide suitable objective evidence to show that their device satisfies the requirements detailed in Annex I of the MDR GSPRs. Where manufacturers determine that specific GSPRs are not applicable to their device, “an explanation as to why [they] do not apply” must be provided, which is a new requirement in the MDR (Annex II, point 4(a)). Moreover, pointing precisely to the key documents demonstrating compliance to each GSPR will simplify the TD review by the NB as well as help you identify possible gaps within your own TD before submission.
Pitfall 6: No implementation readiness documentation of the UDI traceability system
The Unique Device Identification (UDI) system has a direct impact on the labelling, artwork, and DoC, as manufacturers will need to place a UDI carrier on the label of the device and on all higher levels of packaging, except the shipment packaging (Article 27, point 4). Your quality management system will also need to be aligned to the UDI system implementation. Beware to anticipate any risk linked to the UDI system implementation within your Risk Management file.
Pitfall 7: Absence of specific information relating to device design and history
For all classes of medical devices, manufacturers must now provide, as per Annex II, information in the technical documentation to explain the design stages and procedures that are applied to their device (Annex II, point 3). Under the requirements of the MDD/AIMDD, this was only the case for class III devices. Therefore, depending on the classification of the device, manufacturers may need to update the content of the technical documentation.
Pitfall 8: Person Responsible for Regulatory Compliance (PRRC) not designated early enough within your organization
Article 15 of the MDR clearly stipulates the obligation for medical device manufacturers to have available, within their organization (or permanently and continuously at their disposal for micro and small companies), at least one person, possessing the necessary expertise in the field of medical devices, who is responsible for regulatory compliance. Among other responsibilities, the person or people responsible for regulatory compliance must ensure that the technical documentation is compiled and maintained.
Medidee: Easying your MDR transition with services tailored to your needs
From a simple gap analysis to guide you towards the appropriate tests or data reviews needed, to performing those reviews, building test protocols, writing test reports or consolidating the entire TD, Medidee’s team of experts is ready to support you in this process.
Having accompanied many companies in the transition to MDR, Medidee has gained experience and is able to make this transition as smooth as possible for your organization.
Do not wait any longer! Learn more about our MDR Transition service and contact us today to kick-start your own MDR transition!
This article was written by Dr Lydie Moreau.
[ARTICLE] Sampling Strategy: 10 FAQs on Sample Size Selection
Whether you are in the feasibility stage, verifying your design inputs and outputs, or validating your manufacturing process, choosing the wrong sample size for your tests can have a significant impact on your project, especially when this is detected during the market approval phase. Not only will it impact your time-to-market, but you will also likely find yourself repeating expensive testing, or worse, redesigning your product or process if you discover that adequate reliability is in fact not achieved.
Besides the benefits to your product development, sample size selection – including written justification – is also a regulatory requirement. Perhaps the most straightforward regulation is that from the FDA, found in the Quality System Regulation, 21 CFR §820.250(b), which states:
“Sampling plans, when used, shall be written and based on a valid statistical rationale. Each manufacturer shall establish and maintain procedures to ensure that sampling methods are adequate for their intended use... These activities shall be documented.”
Similarly, ISO 13458:2016 §7.3.6 and 7.3.7 state:
“The organization shall document verification … [and] validation plans that include methods, acceptance criteria and, as appropriate, statistical techniques with rationale for sample size.” These are clear requirements to include a written sample size justification in your testing protocols.
While the newly updated EU regulations (MDR/IVDR) do not carry explicit requirements like those of the FDA and in ISO 13485:2016, there is an implied requirement to provide a justification of your sample size selection through the requirements to show compliance with harmonized standards, found throughout the regulations. This includes ISO 13485:2016 in a very broad manner, but can also apply to topic-specific standards, such as those for sterilization or biocompatibility.
It is never too early in your project to start planning for sample size selection, considering its impact on your timelines, the cost of tests or the quality and quantity of representative devices to be produced to that end.
Here are 10 commonly asked questions we get when supporting clients with their sampling strategy.
1. Can’t I just use a sample size of 30 all the time?
First and foremost, the sample size you select should give you the information you need. Are you conducting an early feasibility test to inform your product design? Are you conducting design validation on a frozen design? These are very different types of tests which can require different sampling strategies. To get the most out of your testing – which can be costly to repeat – make sure to select an appropriate sample size to get the information you need. While n=30 may work for some tests, it will not always be the best sample size to meet your needs.
Besides type test (a test on one representative sample of the device with the objective of determining if the device, as designed and manufactured, can meet its requirements), some product-specific standards may include predefined sample size. In all the other cases, a statistical rationale shall be developed.
2. What exactly is a Confidence Level?
How do you know if your test results are meaningful? Choosing a sample size which is too small can leave the story of your testing incomplete – like trying to see the finished image of a puzzle without all the pieces in place. You can think of your confidence level like a 100-piece puzzle: how many pieces of the puzzle do you need to correctly guess the image? The more pieces you have, the more sure you can be about what the image is. The same is true for confidence level – a higher percentage will give you greater assurance that your test results are correct.
3. How do I pick the right Confidence Level?
To determine your confidence level, you need to understand the impact of your results being wrong in order to understand the importance of your results being correct. This is often tied to the product development stage your testing is being conducted in, as well as the risk of “false positives”. Another practical concern is the cost of samples and the time to produce them. While this is an important consideration for your overall project – it cannot be the driving factor and should instead be a secondary consideration when determining sample size. While a low confidence level may seem appealing due to the lower number of required samples, the result may not give you the information you need.
4. Do I need to select a Reliability too?
In short, for design verification, yes. Reliability is a measure of how well a device or product will perform under a certain set of conditions for a specified amount of time. It is an indication of the consistency of the measurement and is critical to specify when selecting a sample size. Like selecting a confidence level, the reliability is related to the risk of the test results being incorrect. Reliability criteria which is too low – leading to a smaller sample size – may lead to test results which do not appropriately reflect the acceptable failure rate of your device.
5. Does it matter what type of test I’m conducting?
The type of test can impact the sample size. It is important to consider if your samples will undergo cyclic testing, if they require real-time or accelerated aging, if the samples will be tested to failure, and even if they need to be representative of different lots, shifts, or manufacturing sites. All of these details and more can inform your sample size selection and how the sample size is defined. For example, if a device is produced at two separate manufacturing sites, careful consideration must be taken to determine if the sites should be sampled together or separately. A similar grouping determination should be made with samples that go through cleaning, sterilization, or aging.
6. Do I need to link my sample size to my risk assessment?
As already discussed, your sample size selection must be informed by the risk of your test results being incorrect or incomplete. However, it should also be tied to the device risks. A pre-determined matrix connecting risk levels and confidence and reliability can add consistency into your sampling process. More severe risks with a higher probability of occurring generally require a higher confidence and reliability level and therefore a higher sample size. Linking these in your sampling procedure can help define your sample size justification.
7. How do I determine which risks are impacted?
In order to link your sample size to your risk assessment, you must determine which risks are related to the test you are conducting. First, identify what the test is challenging – is it a design validation test which is intended to confirm a specific feature of the design? Or perhaps a process validation which will serve in place of an inspection step? In some cases, the failure of the test itself will be directly linked to a risk in your risk analysis. In other cases, the resulting consequence of the failure may be the related risk. In your justification, you can reference the risks which are impacted.
8. Does this approach work for all data types?
The type of data you will receive from your test may help to determine your sample size. Will your test results be pass/fail (or accept/reject)? Will you be testing a numerical value against a one- or two-sided specification? Are you conducting a comparative test between two samples? Depending on the type of data, the method for choosing a sample size may be limited to only one of several different methods and can help you rule out other possible sample size selection processes.
9. How many samples can “fail” and still have an acceptable test?
Now that you’ve determined your confidence and reliability levels, type of test, impacted risks, and data type, the last key parameter to determine is the number of allowable failures. In many cases, the standard is to allow for no failures, or the “c=0” sampling approach. However, this is not the only way to do this. In some cases, particularly depending on the associated risks, it may be acceptable to use a threshold of allowable failures greater than 0. For these situations, it is important to define this in the justification.
10. Any tips on how to reduce my sample size?
Using a statistical approach doesn’t mean you can’t get pragmatic with your sampling plan! There is often more than one way to solve the riddle of sample size selection, with varying results. It can be helpful to talk through testing plans with a cross-functional group to determine the testing – and sample size – which meets your project needs while still using a statistically valid approach. This group can include quality engineers, product designers, manufacturing personnel, and materials experts. The sample size you select must be statistically justified, but that doesn’t mean the methodology, and therefore the answer, is always the same. If your first selection is unreasonably high – for example, you need to test more samples than you produce annually – then go back to the drawing board and think outside the box.
As you can see, developing an adequate sampling strategy requires a strong set of skills, including knowledge of your product, statistics and risk management, all combined with pragmatism and cleverness.
With extensive track record working on similar problematics, Medidee can support you with services ranging from training courses and coaching, up to complete preparation of your sampling strategy.
Contact us today to discuss your project!
This article was written by Paige Elizabeth Sutton.
[TECH LETTER] Medical Device Software incorporating Artificial Intelligence: Generating sufficient evidence under the MDR
Artificial Intelligence (AI) and Machine Learning (ML) technologies have the potential to transform medicine by aiding in the detection, diagnosis, and management of diseases. As digitalization of healthcare generates massive amounts of data, medical device manufacturers are increasingly incorporating AI technologies to automate the analysis of such data targeting to create innovative products and improve patient care. This turn towards AI-enabled medical device software (MDSW) is also evidenced by the plethora of studies evaluating the feasibility of artificial intelligence systems across a wide range of health-related indications.
While the interest in medical applications of AI is strong, inconsistent and incomplete collection of evidence remains one of the barriers to the assessment of the safety and performance of AI-MDSW by regulatory bodies.
According to the provisions of Article 61(1) of the MDR EU 2017/745, it is the responsibility of the manufacturer to specify and justify the level of Clinical Evidence necessary to demonstrate conformity of their medical device to the relevant General Safety and Performance Requirements (GSPRs); this level of clinical evidence should be appropriate in view of the device characteristics and intended purpose.
Determining the appropriate level of evidence might be challenging, especially in the case of AI-enabled MDSW which significantly differs from established medical device software in terms of technical and clinical aspects. At the same time, there is no explicit regulatory guidance for conformity assessment of AI technologies, delineating appropriate and practical evidence generation approaches.
Accordingly, this Technical Letter aims to provide an overview of the considerations for evaluating evidence regarding AI-MDSW.
GET THE TECH LETTER
Please submit the form:
[TECH LETTER] Reprocessing of Medical Devices
Reusable medical devices are devices that healthcare professionals can reprocess and reuse on multiple patients. Inadequate reprocessing between patient uses can result in accumulation of chemical and biological debris. Chemical residues can lead to pyrogenicity issues. Biological debris can lead to microbial proliferation even after cleaning, disinfection and / or sterilization processes. Hence, reprocessing is a critical step to guarantee patient safety and, as such, it is covered in the different regulatory frameworks.
This Tech Letter gives an overview of the reprocessing processes within the US regulatory framework and addresses the importance of adequate reprocessing of medical devices, mentioning the letter issued by FDA regarding the change in reprocessing methods for certain urological endoscopes.
GET THE TECH LETTER
Please submit the form:
[TECH LETTER] Substance-Based Products
Manufacturers producing substance-based products need to qualify their products to be in compliance with the appropriate European legal framework.
This can be challenging, as depending on the mode of action and intended purpose, their product can be regulated by Directive 2001/83/EC relating to medicinal products for human use (MPD) or by the Medical Device Regulation 2017/745 (MDR).
In this Downloadable Techletter, we provide important information that will support you in this process.
GET THE TECHLETTER
Please submit the form:
[TECH LETTER] Cybersecurity requirements
Where does regulatory compliance with cybersecurity requirements begin?
Traditionally, we would rely on state-of-the-art or harmonized standards to demonstrate conformity with such GSPRs, but things quickly complicated, since it is not entirely clear, which standards represent the state-of-the-art for security of medical and IVD devices.
This applies to both the security-related processes at the company level, as well as security capabilities of the device itself.
In this Downloadable Techletter, we discuss a short list of key sources for both knowledge and application of fundamental security concepts to the lifecycle of medical and IVD devices.
GET THE TECHLETTER
Please submit the form:
[TECH LETTER] Spare parts, repair, refurbishment vs. “fully refurbished”
A recurring question related to the repair of devices is whether external, well-qualified, repair shops may pursue their device reparation activities under the MDR.
Where lies the boundary between “spare part” and “accessory for a medical device? When is repair considered equivalent to “fully refurbishing”?
This Downloadable Techletter aims at clarifying the definitions for these terms and determining the possibilities that remain for healthcare institutions.
GET THE TECHLETTER
Please submit the form:
[ARTICLE] IEC 60601 standard series
Medical devices are intended to be used in combination with human beings. Ensuring the safe and effective use of such devices is mandatory according to the European Medical Device Regulation (EU 2017/745) and other legislations regulating medical devices worldwide.
Manufacturers of medical devices are obliged to show compliance to technical state-of-the-art. For instance, the standards of the IEC 60601 series applicable to electrical medical equipment, are listed as consensus standards recognized by the U.S. Food and Drug Administration, and constitute harmonized standards according to Medical Device Directive 93/42/EEC, soon to be also harmonized under MDR. Compliance “can” be demonstrated with the help of the IEC 60601 series of collateral and particular standards for medical electrical equipment. To give more sense to the word “can”, it would be difficult to justify deviation from these standards to a regulatory authority such as a Notified Body, or the FDA.
What are the IEC 60601 standard series?
The IEC 60601 standard series consists of several collateral (horizontal standards like IEC 60601-1-XX which define general requirements for safety and performance) and particular (vertical standards like IEC 60601-2-XX which define product-related requirements) standards and refers to process standards which need to be considered during the different life cycle phases of a medical device. It shall be emphasized that the requirements of applicable particular standards take priority over the general standards, for instance IEC 60601-1.
Medical electrical equipment needs to be evaluated according to IEC 60601-1 general requirements for basic safety and essential performance, IEC 60601-1-2 electromagnetic disturbances – requirements and tests, and IEC 60601-1-6 for usability. Further applicable product standards need to be selected depending on the intended use, intended user and intended use environment of medical electrical equipment.
The IEC 60601-1 standard series is strongly linked to ISO 14971 for application of risk management to medical devices. If the medical electrical equipment uses software, IEC 62304 is applicable as well. IEC 62366-1 for application of usability engineering to medical devices needs to be considered as part of the usability process in combination with IEC 60601-1-6. ISO 14971, IEC 62304 and IEC 62366-1 are so-called process standards to ensure the right process level to guarantee safe and effective medical electrical devices.
Beside the above-listed standards, there is a huge variety on standards for components which will be used within the medical electrical equipment, such as:
And many more…
To meet the general requirements for implementing components in a medical electrical equipment the Conditions of Acceptability (CoA) of such components needs to be considered and verified in the end use of the medical electrical equipment. Considerations for the testing to demonstrate compliance with the IEC series of standards are illustrated through two business cases described below.
Business case 1 - IEC 60601-1 Electrical Safety for a key component of the product
A medical device manufacturer would like to integrate Switch Mode Power Supply (SMPS) in a medical electrical equipment as power supply. Selection of SMPS is important depending on the construction of the Medical Electrical Equipment (MEE). Based on the intended use, intended environment and operational modes of the MEE the following aspects need to be considered for introduction of SMPS:
As a consulting firm, Medidee usually participates at this stage in different ways. On one side, Medidee helps the medical device manufacturer to identify and define the correct specifications, not only in terms of electrical performance, but also in terms of coherence of the options above. Secondly, Medidee assists in making sure that coherence between the different part of the regulatory documentation is maintained along the design and approval process and, also, that possible changes made along the way are handled correctly.
During the certification process with an external ISO/IEC 17025 accredited testing laboratory, relevant tests to verify the applicable requirements will be conducted with the Equipment Under Test (EUT). In this context, Medidee is there to independently support the legal manufacturer of the medical device in the selection and activation of the adequate laboratory.
It is very important to specify requirements in an early development phase to avoid cost intensive design changes during the certification phase of the device. It should be kept in mind that SMPS is part of the insulation diagram and list of critical components of MEE. Furthermore, the electrical and electromagnetic compatibility risk of a MEE needs to be evaluated within the risk management file according to ISO 14971.
Business case 2 - IEC 60601-1-2 EMC testing
A medical device manufacturer would like to initiate certification according to IEC 60601-1-2 to introduce the MEE into the market. First of all, the manufacturer needs to identify which clauses of the standard are applicable for the EUT. Secondly, the medical device manufacturer needs an external ISO/IEC 17025 accredited testing laboratory which has the standard within its scope of testing. According to clause 6.2 Test plan of IEC 60601-1-2, a detailed test plan shall be provided to the test laboratory.
Annex G of IEC 60601-1-2 provides recommendations regarding the minimum test plan contents. However, this information is quite cryptic for non-specialists. This is where Medidee contributes by ensuring the right options and test conditions are selected.
An important aspect for correct EMC testing is the evaluation of operational modes for testing and classification of essential performance. The test requirements can be structured in electromagnetic emission and electromagnetic immunity, as shown below:
Electromagnetic emission – “how does the medical device perturbate the others”:
Electromagnetic immunity: – “how can the medical device be perturbed by the others”
HOW MEDIDEE CAN HELP
Medidee has extensive expertise in the IEC 60601 series and assists manufacturers in identifying the applicable standards and clauses, and in demonstrating compliance with the relevant requirements during different life cycles phases of the medical electrical equipment.
In addition, Medidee supports the communication with external accredited testing laboratories and notified bodies to ensure a smooth certification process of the medical electrical equipment.